You Are At A Restaurant Eating Lunch With A Friend.
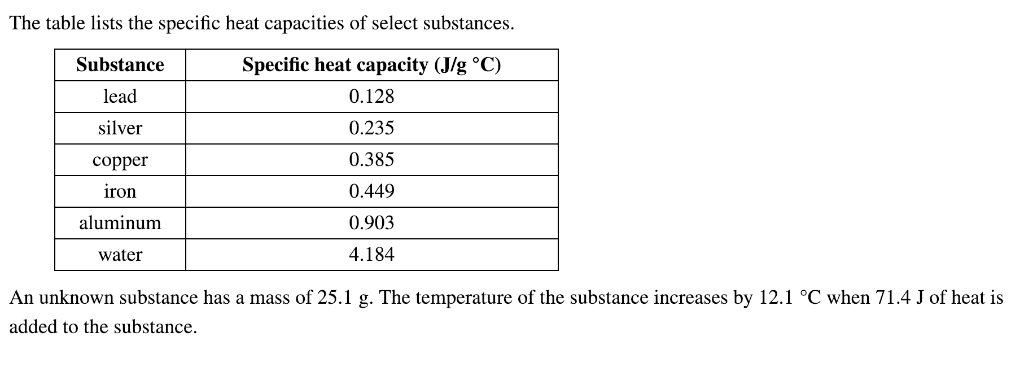
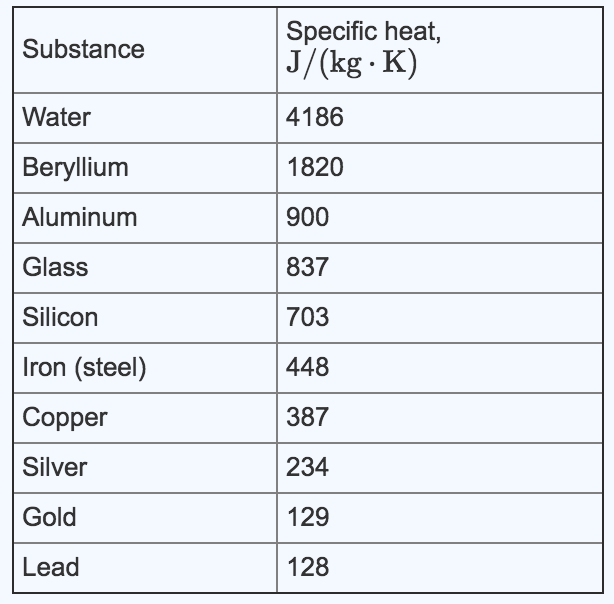
Specific heat capacities for various substances such as lead, lithium, mercury, and methanol are listed. A table chart of specific heat capacities for metals, including lead, is also provided. In addition, the specific heat of lead, its latent heat of fusion, and latent heat of vaporization are mentioned. The overall specificity of heat capacities in various materials, including lead, is discussed.
To find the specific heat of the metal your friend gave you, we can use the principle of conservation of energy and the equation for heat transfer.
The heat lost by the silver piece when it cools down from 100°C to the final temperature is equal to the heat gained by the lead-water system when it warms up from 20.2°C to the final temperature.
The equation for heat transfer is Q = mcΔT, where Q is the heat transferred, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
Let's denote the specific heat of the metal as c_s, the mass of the metal as m_s, the specific heat of lead as c_l, the mass of lead as m_l, and the specific heat of water as c_w. The temperature change for the metal is ΔT_s = 100°C - 22.2°C, and the temperature change for the lead-water system is ΔT_lw = 22.2°C - 20.2°C.
The heat lost by the silver piece is Q_lost = m_s * c_s * ΔT_s, and the heat gained by the lead-water system is Q_gained = (m_l + m_w) * c_lw * ΔT_lw, where c_lw is the specific heat of the lead-water system and m_w is the mass of water.
We have the equation: m_s * c_s * ΔT_s = (m_l + m_w) * c_lw * ΔT_lw
By substituting the known values, we can solve for the specific heat of the metal. But first, we need to find the specific heat of lead, which I will look up for you.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
