when does a medical device manufacturer have to report a
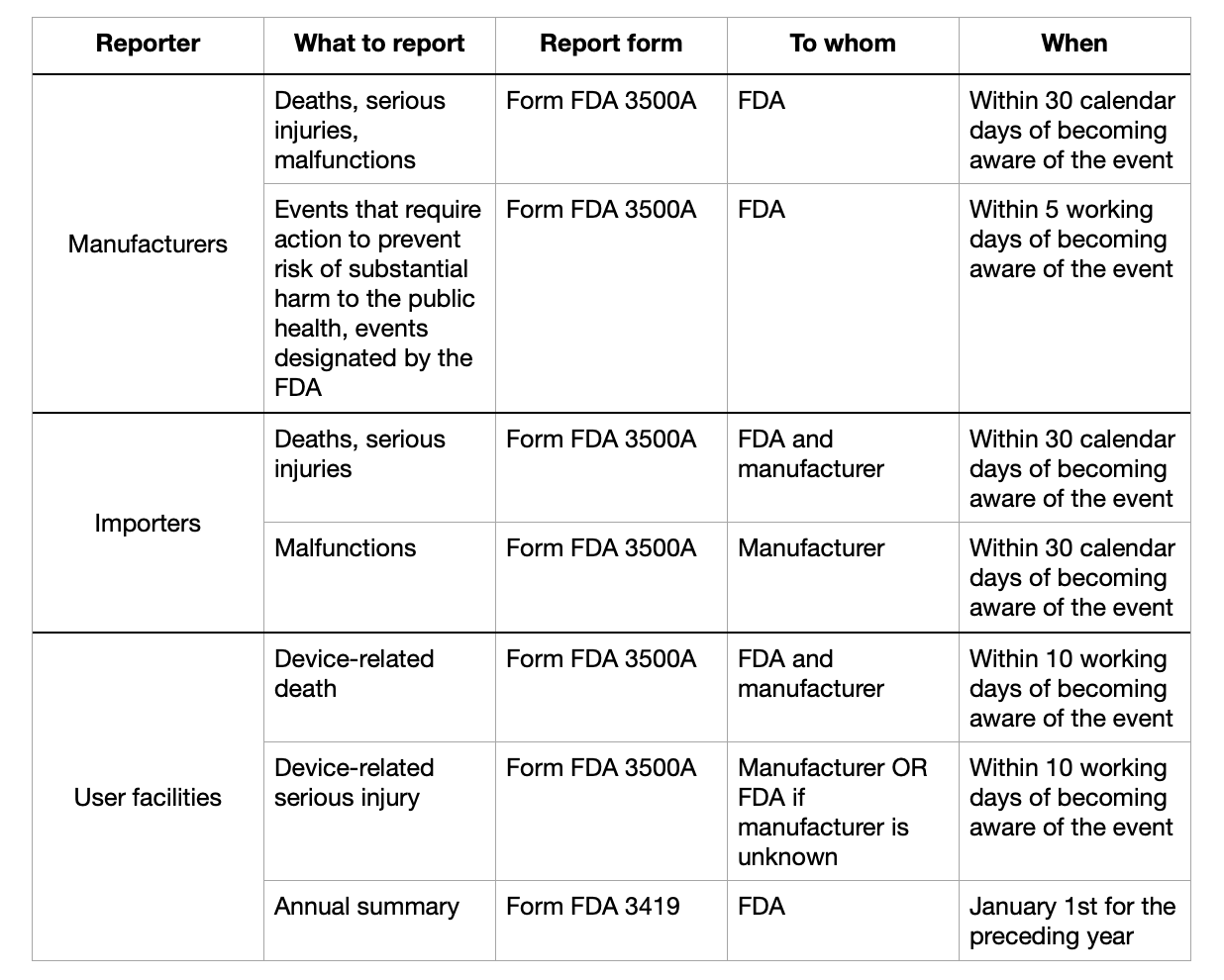
Under the FDA regulations, medical device manufacturers have specific reporting obligations when it comes to known deaths associated with their devices. Here's when a manufacturer must report a known death to the FDA:
-
Timeline for Reporting: Manufacturers are required to report a known death related to their medical device within 30 calendar days of becoming aware of the event. This is stipulated under the Medical Device Reporting (MDR) regulations codified in 21 CFR Part 803.
-
Criteria for Reporting: A known death refers to an incident where the manufacturer has reason to believe their device may have caused or contributed to the death. The obligation to report includes not only deaths directly linked to the device but also those where the manufacturer suspects a possible connection.
-
Submission Process: Reports must be submitted electronically via the FDA's MDR reporting system, unless the manufacturer qualifies for a waiver.
-
User Facility Obligations: If a user facility becomes aware of a device-related death, they are also required to report the incident not only to the FDA but also to the manufacturer.
For more in-depth information and specific guidelines on how to report, you can refer to the FDA's official guidance here and the relevant section of the eCFR here.
Sources
%20decision%20tree_Greenlight%20Guru.png)
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
