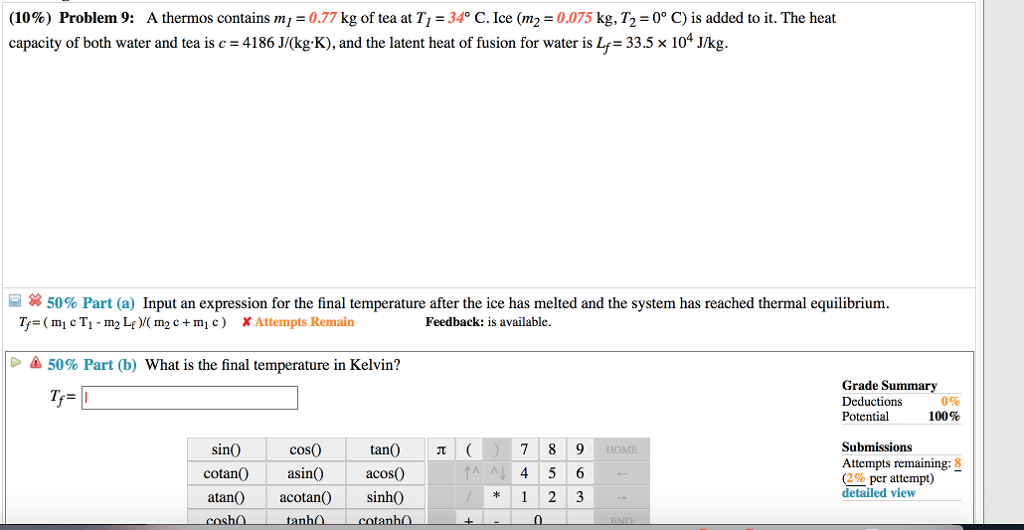
What Is The Final Temperature In Kelvin?
The heat capacity of water and tea is 4186 J/(kg.K) and the latent heat of fusion for water is 33.5 x 10^4 J/kg. When ice is added to the mixture, the specific heat of ice (2000 J/kg°C) and the temperature (0°C) should also be taken into consideration. The mass of the ice is 0.095 kg. The specific heat and temperature for different substances are important in calculating the overall heat of the system. In the Victoria County Station, the ESP Application and Site Safety Analysis Report are essential in analyzing the safety of the site. In a study, LNU95 is found to have a heat capacity of 13985.16 J/(kg-K), making it an important factor in understanding the site's safety.
The final temperature, calculated using the given formula, is approximately 10.57 degrees Celsius. If you need the temperature in Kelvin, you can simply add 273.15 to the result, which gives us approximately 283.72 Kelvin.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
