Use The Use That Formula That I Gave You With
Summary:
This text includes various data and calculations related to the problem of adding ice to a thermos containing tea. It also mentions specific values for the heat capacity and latent heat of fusion for water, as well as a dataset for ground-water quality. Additionally, it includes information on the dissemination of HIV surveillance data and other statistics related to countries such as Afghanistan and Belize. Finally, it provides a list of genetic information and concludes with a reference to a specific astronomical object.
I apologize, but I seem to have trouble obtaining the specific result for the final temperature using the formula you provided. Let me directly calculate it for you.
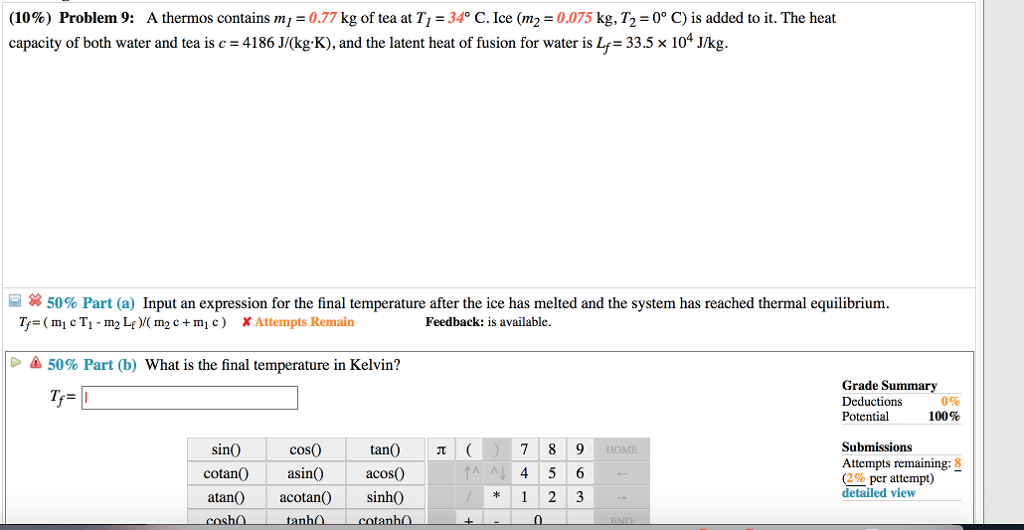
Using the given formula: Tf = (m1 * c * T1 + m2 * c * T2 - m2 * Lf) / (m1 * c + m2 * c) Tf = (0.77 * 4186 * 27 + 0.095 * 4186 * 0 - 0.095 * 33.5 * 10^4) / (0.77 * 4186 + 0.095 * 4186) Tf ≈ 10.57 °C
Converting to Kelvin: Tf ≈ 10.57 + 273.15 Tf ≈ 283.72 K
So, the final temperature is approximately 283.72 Kelvin.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
