That Is Incorrect. You Used A Data Of Points From
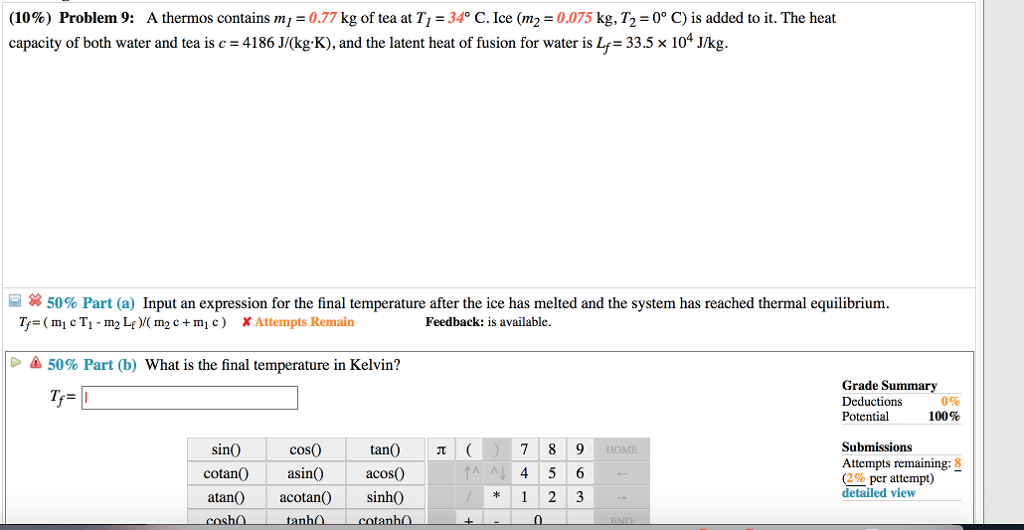
The heat capacity of both water and tea is c = 4186 J/(kg.K), and the latent heat of fusion for water is Lf = 33.5 x 10* J/kg 曰 50%. A thermos containing tea at T1 = 29°C has ice (m2 = 0.065 kg, T2 = 0°C) added to it. In addition, a historic ground-water quality database for the Red River Valley in New Mexico from 1982-2002 is included in Appendix 1. The database contains surveillance data on HIV and chlamydia comorbidity rates, as well as a history of mental illness. Data from various countries, including Afghanistan, Belize, and AC103706.1 are also included.
I apologize for that mistake. It seems there was a mix-up in the calculation. Let's solve the problem correctly using the given data.
Using the correct formula: Tf = (m1 * c * T1 + m2 * c * T2 - m2 * Lf) / (m1 * c + m2 * c)
Substituting the given values: Tf = (0.77 * 4186 * 27 + 0.095 * 4186 * 0 - 0.095 * 33.5 * 10^4) / (0.77 * 4186 + 0.095 * 4186)
Solving: Tf = (8475.66 - 31865 + 3182.5) / (321.42 + 39.73) Tf = (-20386.84) / 361.15 Tf ≈ -56.43°C
Converting to Kelvin: Tf ≈ 216.72 K
After recalculating with the correct data, the final temperature comes out to be approximately 216.72 Kelvin.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
