Please Also Insert References To These Questions: 1. Scale up Readiness:
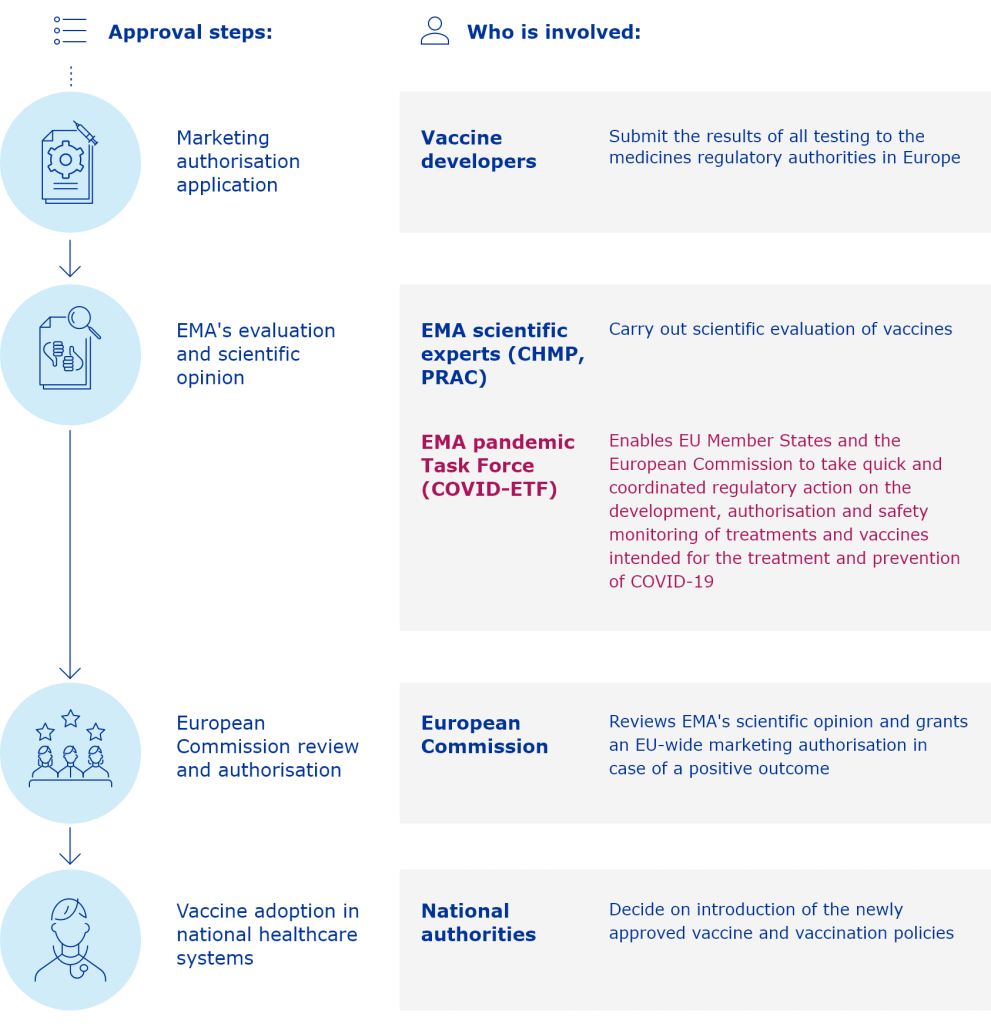
The European Medicines Agency (EMA) has published updated scientific guidelines on vaccines to aid in the preparation of marketing-authorisation applications. These guidelines were revised in April 2022 and provide further clarification on recording patients' vaccine dose schedules and indications for drugs. EMA has also launched CMC flexibilities to support fast-track drug development for the public to access quality medicine. This review discusses key enablers for accelerating drug development and preparedness, focusing on CMC strategies and technical challenges. It is important to understand these challenges in order to successfully develop a CMC dossier, as CMC requirements are not waived for COVID-19 vaccines in the EU. Industry reflections in this paper also provide recommendations for CMC and GMP approaches to support the development of new COVID-19 medicines. EMA's “toolbox guidance” addresses the need for quality data packages for priority medicines, including those targeting unmet needs. EMA's data requirements for vaccines targeting new virus variants assume the fundamental importance of CMC processes.
Here are the references to specific guidelines from the EMA for the questions you've provided:
-
Scale-Up Readiness:
- EMA's "Guideline on process validation for finished products" and "Guideline on process validation for the pharmaceutical industry" provide insights into ensuring a smooth scale-up of production and identification of potential challenges with mitigation strategies.
-
Raw Material Management:
- EMA's "Guideline on good manufacturing practice for active pharmaceutical ingredients" and "Good Distribution Practice of Medicinal Products for Human Use" cover strategies for ensuring a stable supply of critical raw materials, as well as managing fluctuations in raw material quality or availability.
-
Further Process Optimization:
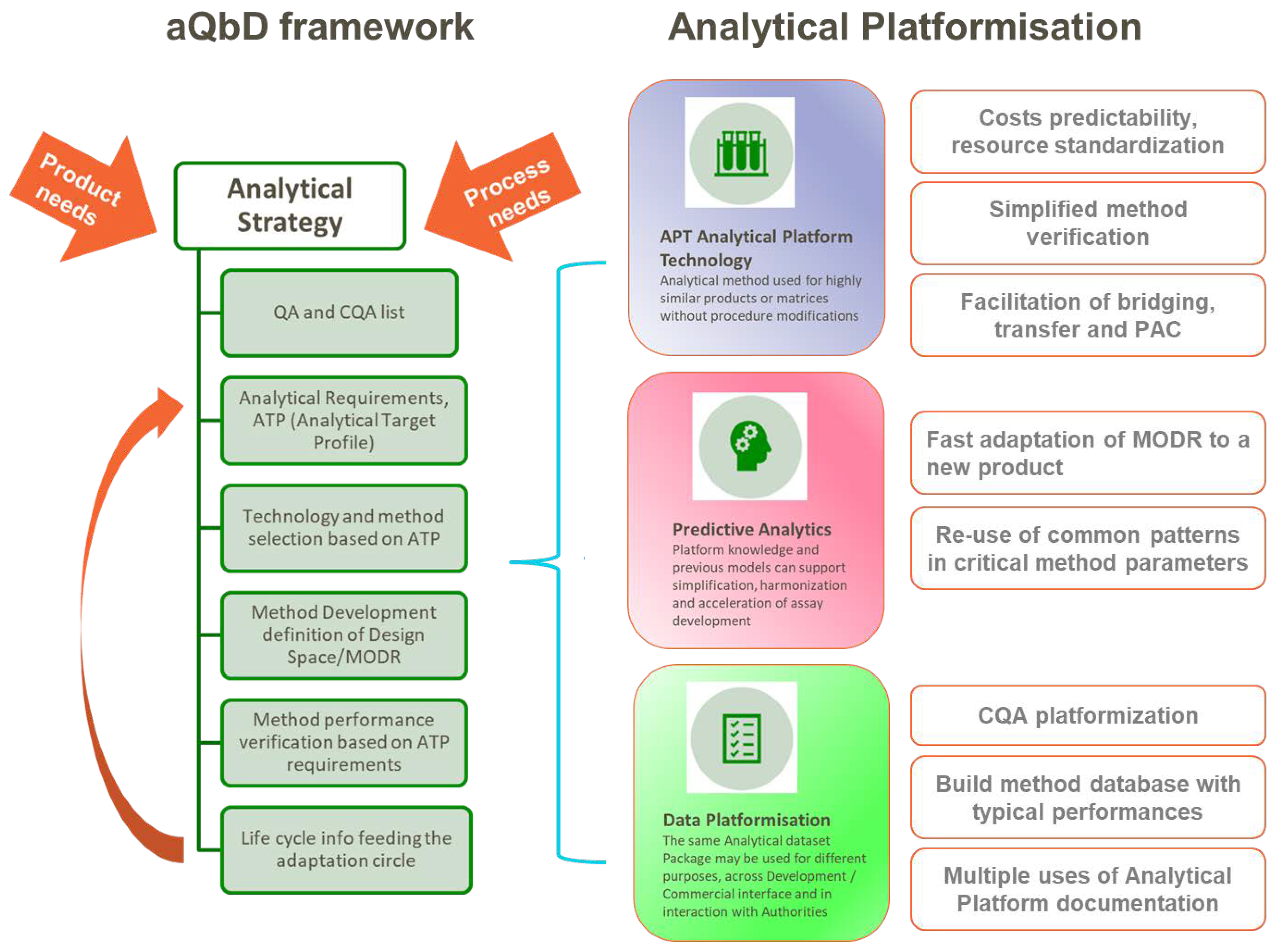
- Opportunities for process optimization can be explored using the EMA's "Guideline on Process Validation for Finished Products" and "ICH Q8 (R2) Pharmaceutical Development."
-
Validation for Commercial Production:
- Additional validation studies required for commercial-scale production are detailed in the EMA's "Guideline on process validation for finished products" and "Guideline on good manufacturing practice for medicinal products for human and veterinary use."
-
Regulatory Strategy for Phase 3 and Commercialization:
- The EMA's "Guidelines for Regulatory Interaction Between Regulatory Authorities and Applicants" and "Guidance for pharmaceutical development of medicines for use in the paediatric population" can provide an overview of regulatory interactions and requirements.
-
Quality Control and Assurance:
- Information on maintaining and enhancing quality control and assurance is available in the EMA's "Guideline on Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use" and "Guideline on Process Validation for Finished Products."
-
Packaging and Labeling:
- The current packaging and labeling strategy can be assessed with respect to EMA's "Guideline on Packaging Information for Medicinal Products" and "Guideline on the Packaging Information of Medicinal Products for Human Use."
-
Post-Market Surveillance Planning:
- EMA's "Guideline on good pharmacovigilance practices (GVP)" and "Guideline on good pharmacovigilance practices: Module IX—Signal Management" provide insights into post-market surveillance planning and real-world data collection for ongoing safety monitoring.
These guidelines offer in-depth information and specific regulatory expectations for various aspects of the pharmaceutical development and regulatory submission process.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
