give information about tirzepatide: mechanism of action, cientific studies and
Tirzepatide is an innovative medication designed for the management of type 2 diabetes. It functions as a dual receptor agonist, specifically targeting the glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors. This unique mechanism enhances insulin secretion in response to meals while simultaneously reducing glucagon levels, which lowers glucose production in the liver. As a result, tirzepatide not only promotes effective glycemic control but also aids in weight management by helping patients feel fuller after eating.
For more in-depth information on its mechanism of action, you can refer to several resources. The American Diabetes Association provides an overview in their publication here. Additional details can be found on DrugBank, which elaborates on the insulin secretion stimulation and glucose-lowering effects of tirzepatide here. The unique activities it possesses to stimulate insulin secretion are discussed in an article from Duke Health here. Furthermore, a thorough explanation of its dual agonist properties and receptor affinities is available on Wikipedia here.
In terms of scientific studies, tirzepatide has undergone various clinical trials to evaluate its efficacy and safety. Results have shown significant improvements in glycemic control and weight loss when compared to traditional therapies. It is critical to consider such scientific evaluations to understand the compound's impact fully.
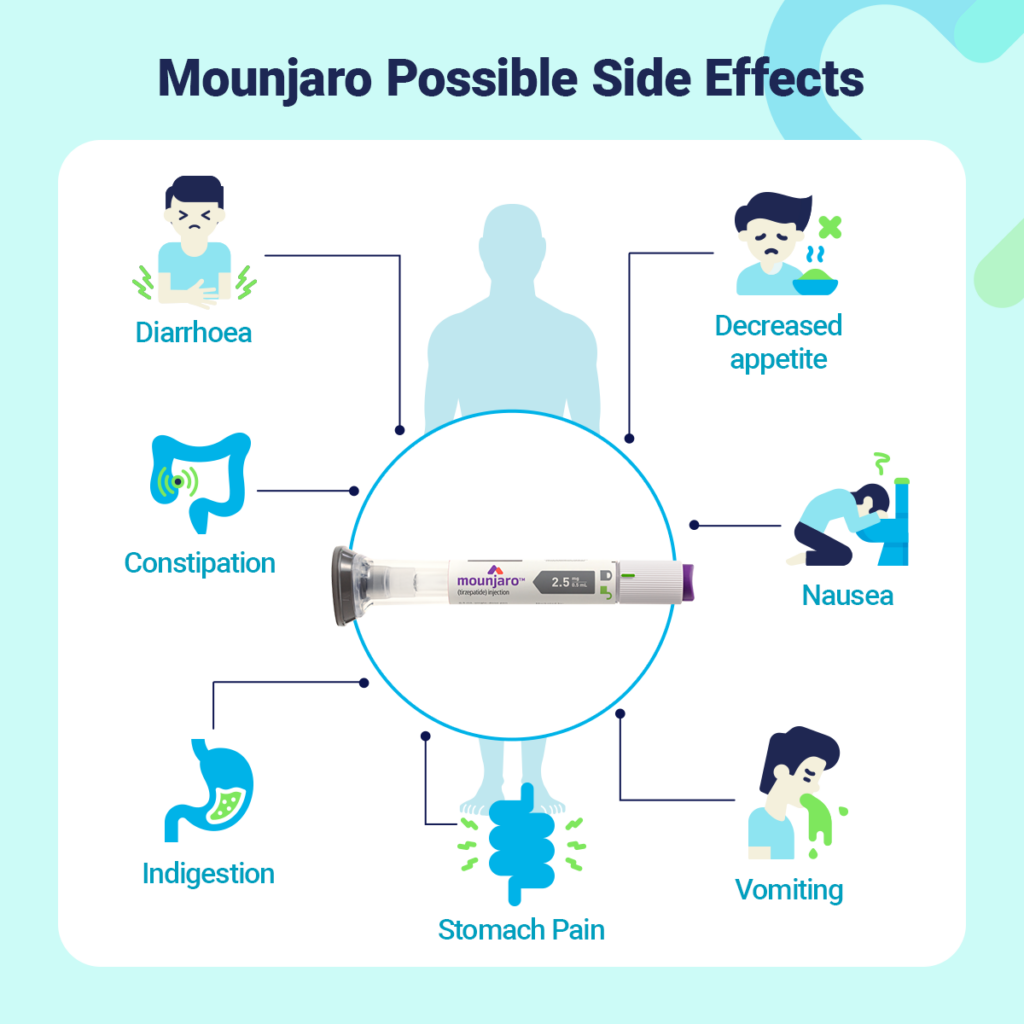
Regarding side effects, tirzepatide may induce gastrointestinal issues, such as nausea, vomiting, diarrhea, and decreased appetite. Other potential adverse effects include possible hypoglycemia, especially when taken in combination with other diabetes medications. A complete list of side effects and contraindications should be reviewed by healthcare providers to determine its suitability for individual patients.
In summary, tirzepatide represents a significant advancement in diabetes treatment, characterized by its dual receptor targeting mechanism, effective clinical outcomes, and manageable side effects. For comprehensive knowledge about this compound, including potential risks, consulting healthcare professionals and accessing peer-reviewed medical literature is strongly recommended.Tirzepatide: Overview, Mechanism of Action, Scientific Studies, and Side Effects
Mechanism of Action
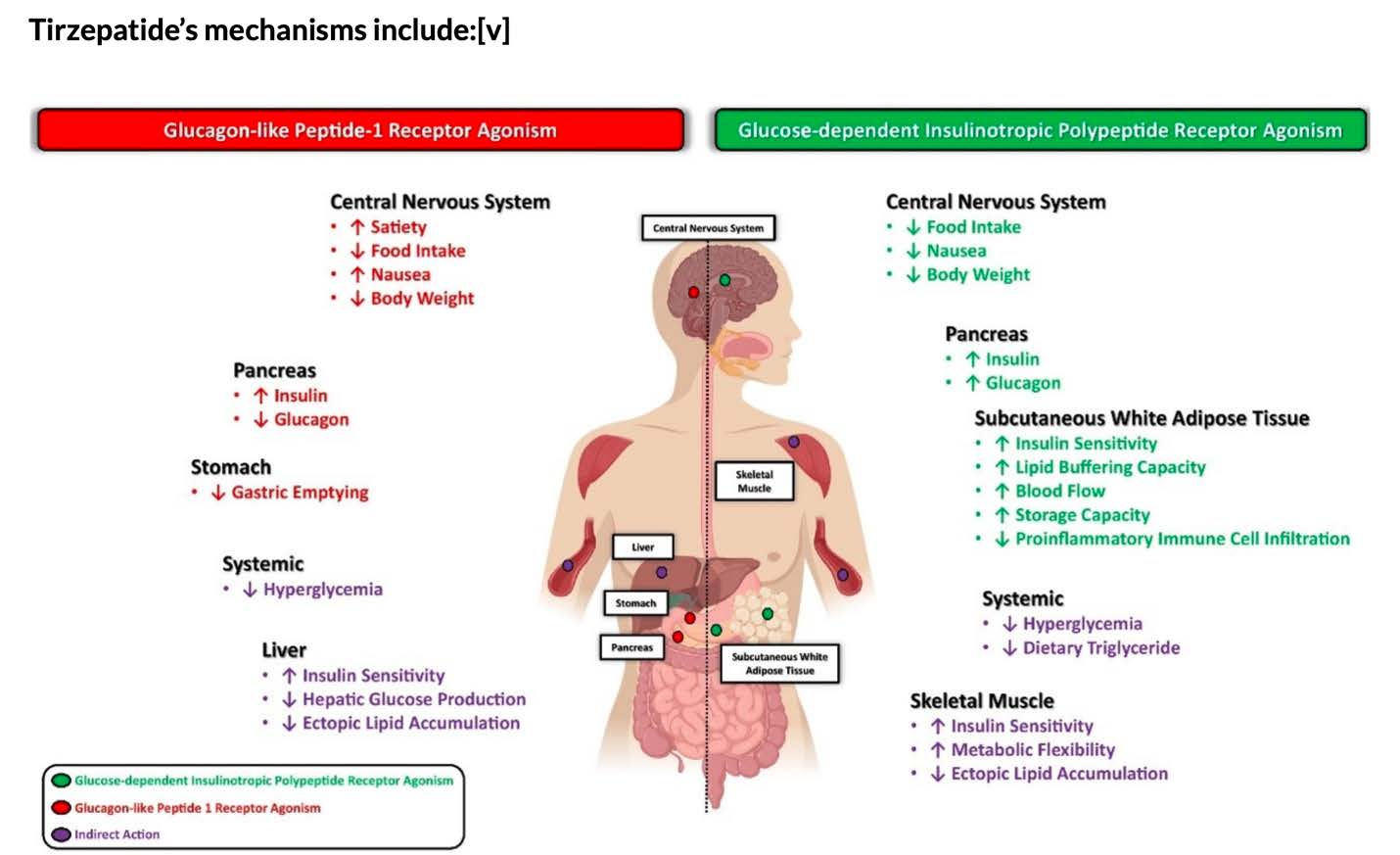
Tirzepatide is a novel injectable medication that functions as a dual agonist for two key receptors involved in glucose metabolism: the glucagon-like peptide 1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP) receptor. As a synthetic peptide, it stimulates insulin secretion in a glucose-dependent manner and reduces glucagon levels, which contributes to lower blood glucose levels. Additionally, tirzepatide aids in inducing satiety, thereby helping to control appetite post-meals. Importantly, it has been noted that tirzepatide shows a greater affinity for GIP receptors compared to GLP-1 receptors, which is part of its unique therapeutic action (American Diabetes Association, Wikipedia, DrugBank).
Scientific Studies
Several pivotal studies have evaluated the effectiveness of tirzepatide for managing obesity and type 2 diabetes. The SURMOUNT-1 trial investigated its efficacy in adults with obesity who do not have diabetes, highlighting significant weight loss outcomes (NEJM). Additional research has demonstrated that tirzepatide is effective in producing weight reductions of 5%, 10%, and even 15% compared to other medications (PMC). The medication also showed improvements in beta-cell function and insulin sensitivity in individuals with type 2 diabetes (NEJM). A recent clinical study confirmed that tirzepatide could safely reduce body mass index (BMI), waist circumference, and body weight significantly, showcasing its promise as a treatment option for obesity (Frontiers in Public Health).
Side Effects
Common side effects associated with tirzepatide include gastrointestinal issues such as nausea, vomiting, diarrhea, and constipation. These are typically mild to moderate and tend to diminish over time as the body adapts to the medication. More serious side effects may include pancreatitis, kidney problems, and allergic reactions, although such instances are less frequent. It is recommended that individuals considering tirzepatide review these potential side effects and consult with healthcare providers to assess personal risk factors and develop a comprehensive treatment plan.
For further information and deeper insights into tirzepatide’s action, studies, and side effects, additional reading materials can be accessed through various links provided, including details from reputable medical sources and studies.### Tirzepatide: Overview
Mechanism of Action
Tirzepatide is a novel injectable medication that acts as a dual agonist for the glucagon-like peptide 1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP) receptor. This unique mechanism allows tirzepatide to enhance insulin secretion in response to elevated blood glucose levels while simultaneously reducing glucagon levels, which can help decrease hepatic glucose production. Additionally, it has been shown to increase feelings of satiety after meals, contributing to its efficacy in weight management (American Diabetes Association, link; DrugBank, link; GoodRx, link).
Tirzepatide is noted for having a greater affinity for GIP receptors compared to GLP-1 receptors, and its dual receptor activity has been linked to superior glycemic control and weight loss outcomes compared to therapies targeting only one receptor (Wikipedia, link; Duke Health, link).
Scientific Studies
Clinical research has demonstrated tirzepatide's effectiveness in treating obesity and type 2 diabetes. The SURMOUNT-1 trial highlighted its efficacy in weight management for adults without diabetes, showing substantial weight loss (NEJM, link). Another systematic review affirmed that tirzepatide led to greater reductions in body weight compared to other anti-obesity medications, with patients achieving significant weight milestones (PMC, link).
Further studies have reported that continued administration of tirzepatide resulted in a mean body weight reduction of 20.9% in participants over a sustained period (JAMA, link). Research has also indicated improvements in insulin sensitivity and markers of beta-cell function in individuals with type 2 diabetes (NEJM, link; Nature, link).
Side Effects
Common side effects associated with tirzepatide include gastrointestinal disturbances such as nausea, diarrhea, abdominal pain, constipation, and vomiting. These effects are particularly prevalent at the initiation of treatment or during dosage adjustments. Other reported side effects include decreased appetite and gastroesophageal reflux (Drugs.com, link; Cleveland Clinic, link; WebMD, link).
Although the majority of these side effects are temporary, monitoring is advised, especially during the early stages of therapy (Hers, link; Braxton Medical Clinic, link).
In summary, tirzepatide serves as a promising treatment option for weight management and type 2 diabetes, demonstrating a unique dual-action mechanism and a favorable profile in clinical studies. However, attention to its side effects is essential for patient safety and comfort.
Sources
Related Questions
Work fast from anywhere
Stay up to date and move work forward with BrutusAI on macOS/iOS/web & android. Download the app today.
